In news
With an eye on China, India looks at the lithium reserves in Argentina, Chile and Bolivia
Key highlights
In its economic offensive against China, India, through a newly-floated state-owned company, inked a pact with an Argentine firm mid-last year to jointly prospect lithium in the South American country that has the third largest reserves of the silver-white alkali metal
Why does India eye on Lithium reserves?
- Presently, India is heavily dependent on import of these cells and the move to ink sourcing pacts for lithium is seen as another salvo in the front against China, a key source of both the raw material and cells.
- India is seen as a late mover as it attempts to enter the lithium value chain, coming at a time when EVs are predicted to be a sector ripe for disruption.
About Lithium
Lithium (Li), chemical element of Group 1 (Ia) in the periodic table, the alkali metal group, lightest of the solid elements.
The metal itself which is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Three fragments of Lithium metal.
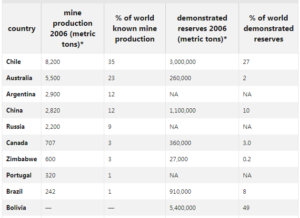
Top ten Lithium producing countries
Significant Uses
- The principal industrial applications for lithium metal are in metallurgy, where the active element is used as a scavenger (remover of impurities) in the refining of such metals as iron, nickel, copper, and zinc and their alloys.
- A large variety of nonmetallic elements are scavenged by lithium, including oxygen, hydrogen, nitrogen, carbon, sulfur, and the halogens.
- Lithium is utilized to a considerable extent in organic synthesis, both in laboratory reactions and industrially.
- A key reagent that is produced commercially on a large scale is n-butyllithium, C4H9Li. Its principal commercial use is as an initiator of polymerization, for example, in the production of synthetic rubber.
- It is also extensively used in the production of other organic chemicals, especially pharmaceuticals.
- Because of its light weight and large negative electrochemical potential, lithium metal, either pure or in the presence of other elements, serves as the anode (negative electrode) in many nonrechargeable lithium primary batteries.
- Since the early 1990s much work has been done on high-power rechargeable lithium storage batteries for electric vehicles and for power storage.
- The most successful of these provides for separation of the anode and a cathode such as LiCoO2 by a solvent-free conducting polymer that permits migration of the lithium cation, Li+. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices.
- It is a crucial building block of the lithium-ion rechargeable batteries that power electric vehicles (EVs), laptops and mobile phones.
- Lightweight lithium-magnesium alloys and tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and other industries.
- Metallic lithium is used in the preparation of compounds such as lithium hydride.
















