In news
Department of Biotechnology, Ministry of S&T supported First CAR-T cell therapy conducted at ACTREC, Tata Hospital in Mumbai
Need for The Chimeric Antigen Receptor T-cell (CAR-T) therapy
- CAR-T therapy has emerged as a breakthrough in cancer treatment.
- Clinical trials conducted globally have shown promising results in end stage patients, especially in patients suffering from Acute Lymphocytic Leukemia.
- At present this technology is not available in India because each patient’s CAR-T cell therapy costs 3-4 crore (INR).
- The challenge therefore is to develop this technology in a cost-effective manner and make it available for the patients.
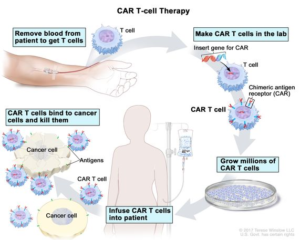
Chimeric antigen receptor T cells are T cells that have been genetically engineered to produce an artificial T-cell receptor for use in immunotherapy.
Chimeric antigen receptors are receptor proteins that have been engineered to give T cells the new ability to target a specific protein. The receptors are chimeric because they combine both antigen-binding and T-cell activating functions into a single receptor.
Collaboration to develop CAR-T cell technology
- In order to promote and support development of CAR-T cell technology against cancer and other diseases, BIRAC and DBT have taken initiatives and launched specialized calls to invite proposals in the last 2 years.
- Recently,the first CAR-T cell therapy (a type of gene therapy) was done at the Bone Marrow Transplant unit at ACTREC, Tata Memorial Center in Mumbai.
- The CAR-T cells were designed and manufactured at Bioscience and Bioengineering (BSBE) department of IIT Bombay.
- This work is partly supported by BIRAC-PACE scheme.
- The TMC-IIT Bombay team are further supported to extend this project for conducting Phase I/II trial of their CAR-T product by DBT/BIRAC, through the National Biopharma Mission.
- This is a “first in India” gene therapy in early phase pilot clinical trial and the dedicated efforts and excellent collaboration between IIT Bombay and Tata Memorial Hospital, Mumbai.
- The central government’s National Biopharma Mission-BIRAC has approved 19.15 Cr crore to the team for conducting a first-in-human phase-1/2 clinical trial of the CAR-T cells.
- The clinical trials are being done by Dr Gaurav Narula, Professor of Paediatric Oncology and Health Sciences, and his team from TMC, Mumbai, and the novel CAR-T cells that will act as drugs that were manufactured by Prof Rahul Purwar, Bioscience and Bioengineering (BSBE) department and his team at IIT Bombay.
- The design, development, and extensive pre-clinical testing was carried out by IIT-B as a collaborative project with Tata Memorial Center, Mumbai by the two InvestigatorsCAR-T) therapy
Other initiatives of National Biopharma Mission regarding T cells
- It is also supporting the development of Lentiviral vector manufacturing facility for packaging plasmids used to transfer the modified T cell inside the body, cGMP facility for T-cell transduction and expansion for CAR T-cell manufacturing to two other organizations.
- The development of CAR-T cell technology for diseases including acute lymphocytic leukemia, multiple myeloma, glioblastoma, hepatocellular carcinoma and type-2 diabetes is supported through DBT.
Extra Reading: https://journalsofindia.com/national-biopharma-mission/
















