In news– Even though the night sky is dark, due to airglow it illuminates with a mixture of red, yellow, and green colours.
What is airglow?
- Airglow occurs when atoms and molecules in the upper atmosphere, excited by sunlight, emit light to shed their excess energy. Or, it can happen when atoms and molecules that have been ionized by sunlight collide with and capture a free electron.
- In both cases, they eject a particle of light — called a photon — in order to relax again.
- The phenomenon is similar to auroras, but where auroras are driven by high-energy particles originating from the solar wind, airglow is energized by ordinary, day-to-day solar radiation.
- This phenomenon originates with self-illuminated gases and has no relationship with Earth’s magnetism or sunspot activity.
- The Sun produces a broad spectrum of visible light, which we see as white but it includes all the colours of the rainbow.
- When sunlight passes through the air, atoms and molecules in the atmosphere scatter blue light in all directions, far more than red light.
- This is called Rayleigh scattering, and results in a white Sun and blue skies on clear days.
- At sunset we can see this effect dialled up, because sunlight has to pass through more air to reach us.
- When the Sun is close to the horizon, almost all the blue light is scattered (or absorbed by dust), so we end up with a red Sun with bluer colours surrounding it.
- If you look at the night sky, it is obviously dark, but it isn’t perfectly black. Yes, there are the stars, but the night sky itself glows. This isn’t light pollution, but the atmosphere glowing naturally.
- On a dark moonless night in the countryside, away from city lights, you can see the trees and hills silhouetted against the sky.
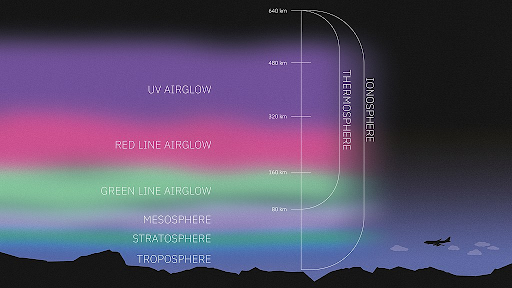
- This glow, called airglow, is produced by atoms and molecules in the atmosphere.
- In visible light, oxygen produces green and red light, hydroxyl (OH) molecules produce red light, and sodium produces a sickly yellow.
- Nitrogen, while far more abundant in the air than sodium, does not contribute much to airglow.
- The distinct colours of airglow are the result of atoms and molecules releasing particular amounts of energy (quanta) in the form of light.
- For example, at high altitudes ultraviolet light can split oxygen molecules (O₂) into pairs of oxygen atoms, and when these atoms later recombine into oxygen molecules they produce a distinct green light.
- Sodium atoms make up a minuscule fraction of our atmosphere, but they make up a big part of airglow, and have a very unusual origin – shooting stars.
- One can see shooting stars on any clear dark nigh. They are teensy tiny meteors, produced by grains of dust heating up and vaporising in the upper atmosphere as they travel at over 11 kilometres per second.
- As shooting stars blaze across the sky, at roughly 100 kilometres altitude, they leave behind a trail of atoms and molecules.
- Sometimes one can see shooting stars with distinct colours, resulting from the atoms and molecules they contain.
- Very bright shooting stars can even leave visible smoke trails. And among those atoms and molecules is a smattering of sodium.