In news–Recently, researchers have shown that it is possible to use light as a fuel to move microbots in real-body conditions with intelligent drug-delivery that is selectively sensitive to cancer cells.
Key highlights of the research-
- The research led by Max Planck Institute for Intelligent Systems (MPI-IS) and Max Planck Institute for Solid State Research (MPI-FKF), Stuttgart, Germany, aims at moving microbots into the bloodstream to deliver drugs.
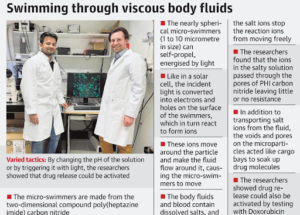
- Made from the two-dimensional compound poly (heptazine imide) carbon nitride (PHI carbon nitride), these microbots or Micro-swimmers are nothing like the miniaturized humans.
- They range from 1-10 micrometer (a micrometer is one-millionth of a meter) in size, and can self-propel when energized by shining light.
- The PHI carbon nitride microparticles are photocatalytic.
- Like in a solar cell, the incident light is converted into electrons and holes, these charges drive reactions in the surrounding liquid.
- The charges react with the fluid surrounding them.
- This reaction, combined with the particle’s electric field, makes the microbots (micro-swimmers) swim.
- As long as there is light, electrons and holes are produced on the surface of the swimmers, which in turn react to form ions and an electric field around the swimmer.
- With light, the researchers not only move the microbots but can direct their motion towards a specific goal.
- The diffusion of the swimming medium in one direction propels the micro-swimmer in the opposite direction. This is like a boat moving in the direction opposite to the oar strokes.
- The particles are nearly spherical, and the incident light illuminates one-half of the sphere, leaving the other dark.
- As photocatalysis is light-driven, it occurs only on the brightened hemisphere. As the ions move from the bright side to the dark side, micro-swimmers march towards the direction of the light source.
- However, all the chemically propelled swimmers can’t swim in solutions containing salts.
- While carbon nitride is an excellent photo-catalyst, the two-dimensional PHI has a sponge-like structure full of pores and voids and charge storage properties.
- The researchers found that the ions in the salty solution passed through the pores of PHI carbon nitride. Thus, there was little or no resistance from the salt ions.
- The voids and pores on the microparticles worked as cargo bays and could soak up large amounts of drug.
- The researchers found that Doxorubicin, a drug used to treat cancer, was readily absorbed. By changing the pH of the solution or by triggering it with light, the researchers showed the drug release could be activated.
















