In News: Nanobodies are a potential therapeutic strategy for treating COVID-19. While many previous studies report nanobodies active against SARS-CoV-2 in vitro, none have been evaluated via animal models’ intranasal administration.
About the Research
- At least one of these nanobodies could prevent infections and detect virus particles by grabbing hold of SARS-CoV-2 spike proteins, the researchers suggest in the journal Scientific Reports.
- This nanobody, called NIH-CoVnb-112, appeared to work equally well in either liquid or aerosol form, which suggests it could remain effective after inhalation.
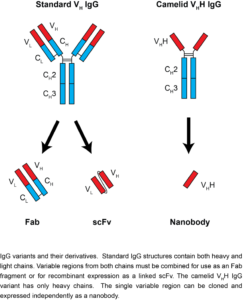
- A nanobody is a special type of antibody naturally produced by the immune systems of camelids, a group of animals that includes camels, llamas, and alpacas. They are called nanobodies because they are tiny, about a tenth the weight of most human antibodies.
- Because nanobodies are more stable, less expensive to produce, and easier to engineer than typical antibodies, researchers have been using them for medical research.
- Since the pandemic broke, several researchers have produced llama nanobodies against the SARS-CoV-2 spike protein that may be effective at preventing infections.
- In the current study, the researchers used a slightly different strategy than others to find nanobodies that may work especially well, NIH said in a statement.
- The spike protein acts like a key during coronavirus infection. It “unlocks” the door to infections when it binds to a human protein called ACE2 receptor on the cell surface.
- The NIH scientists developed a method that would isolate nanobodies that block infections by covering part of the spike protein that bind to and unlock the ACE2 receptor.
Nanobodies
- Nanobodies are a unique kind of monoclonal antibody derived from a camelid IgG variant, consisting of a single heavy-chain variable domain that can bind its antigen as strongly as a standard antibody.
- As nanobodies lack a light chain, they are both significantly smaller than standard antibodies, and have unique flexibility at their antigen-binding interface.
- This combination allows nanobodies to bind in different modes than typical antibodies, covering more chemical space and allowing binding to epitopes otherwise inaccessible to antibodies.
- Nanobodies are also significantly smaller (~15 kDa) and more stable than standard antibodies, and can be easily genetically engineered for additional functionality.