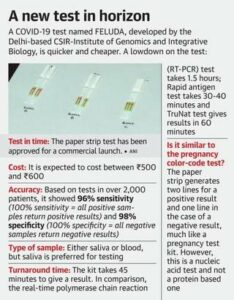
The Tata CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) test, powered by CSIR-IGIB (Institute of Genomics and Integrative Biology) FELUDA, received regulatory approvals today from the Drug Controller General of India (DCGI) for commercial launch, as per ICMR guidelines, meeting high quality benchmarks with 96% sensitivity and 98% specificity for detecting the novel coronavirus.
More About Feluda Test
- Feluda, the acronym for FNCAS9 Editor Linked Uniform Detection Assay, uses indigenously developed CRISPR gene-editing technology to identify and target the genetic material of SARS-CoV2, the virus that causes Covid-19.
- According to CSIR, the test matches accuracy levels of RT-PCR tests, considered the gold standard in the diagnosis of Covid-19, has a quicker turnaround time and requires less expensive equipment.
- It is also the world’s first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus. Other CRISPR tests use CAS12 and CAS13 proteins to detect SARS-CoV2.
- The CSIR research team came about creating the new test kit while working under the sickle cell mission for genome diagnostics and therapeutics.
- CRISPR, short form for Clustered Regularly Interspaced Short Palindromic Repeats, is a gene editing technology and finds its use in correcting genetic defects and treating and preventing the spread of diseases.
- The CRISPR technology can detect specific sequences of DNA within a gene and uses an enzyme functioning as molecular scissors to snip it. It also allows researchers to easily alter DNA sequences and modify gene function.