A fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cell systems are a clean, efficient, reliable, and quiet source of power. Fuel cells do not need to be periodically recharged like batteries, but instead continue to produce electricity as long as a fuel source is provided.
More About Fuel Cells
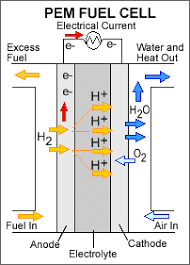
- A fuel cell is composed of an anode, cathode, and an electrolyte membrane. A typical fuel cell works by passing hydrogen through the anode of a fuel cell and oxygen through the cathode.
- At the anode site, a catalyst splits the hydrogen molecules into electrons and protons. The protons pass through the porous electrolyte membrane, while the electrons are forced through a circuit, generating an electric current and excess heat.
- At the cathode, the protons, electrons, and oxygen combine to produce water molecules. As there are no moving parts, fuel cells operate silently and with extremely high reliability.
- Fuel cells that use pure hydrogen fuel are completely carbon-free, with their only byproducts being electricity, heat, and water.
Applications of Hydrogen Fuel Cells
- NASA is a major user of hydrogen resources for its space program. NASA fuels the booster rockets of the space shuttle using liquid hydrogen and employs hydrogen batteries for electrical sources.
- Hydrogen fuel cells can power any portable device that uses batteries. Unlike a typical battery, the hydrogen fuel cell continues to produce energy with the continuous supply of fuel. This ability of the fuel cells enables them to power devices like hearing aids, video recorders, cellular phones and laptop computers.
- Stationary hydrogen fuel cells are the largest and most powerful fuel cells. They are a clean, reliable source of power to cities, towns and buildings. These fuel cells are also used for back-up and remote power applications including remote weather stations and rural locations.
















