In news– Researchers at the Paderborn University, Germany have reported being able to make a unique class of catalysts used in chemistry to accelerate reactions called Lewis super-acids.
About Lewis super acids-
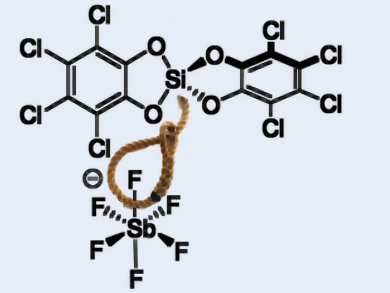
- Named for the chemist, G N Lewis, Lewis super-acids derive from Lewis acids.
- A Lewis acid is any substance, such as a Hydrogen ion (H+) that can accept a pair of nonbonding electrons.
- In other words, a Lewis acid is an electron-pair acceptor.
- A Lewis base is any substance, such as the OH- ion, that can donate a pair of nonbonding electrons. A Lewis base is therefore an electron-pair donor.
- Because Lewis acids add electron pairs, they are often used to speed up chemical reactions.
- Lewis superacids are stronger than antimony pentafluoride, the strongest Lewis acid and can break even the toughest bonds.
- Breaking strong, chemical bonds requires highly reactive substances.
- Because they are so reactive, they are hard to manufacture however the research team, in a paper, said they used a “trick” to produce these super acids.
- Being able to make these super acids, enables non-biodegradable fluorinated hydrocarbons, similar to Teflon, and possibly even climate-damaging greenhouse gases, such as sulphur hexafluoride, to be converted back into sustainable chemicals.
Source: The Hindu